 Silicon occupies a position in the periodic table at the border between metals and insulators, and has important technological applications. At low pressure, crystalline silicon adopts the diamond structure and is an indirect band-gap semiconductor. With increasing pressure, crystalline Si transforms from the diamond phase through a more highly-coordinated metallic β-tin phase, and then to a hexagonal phase. Such polymorphic phase transitions are usually accompanied by interesting changes in electronic properties. Similar to crystalline silicon, amorphous Si is also known to exhibit polymorphism. Interestingly at equilibrium, liquid Si is metallic and is denser than its crystalline counterpart, very similar to the case with water. In spite of the widespread use of Si in industry, the nature of the transition of the dense metallic liquid to an open network semiconducting solid is poorly understood.
Silicon occupies a position in the periodic table at the border between metals and insulators, and has important technological applications. At low pressure, crystalline silicon adopts the diamond structure and is an indirect band-gap semiconductor. With increasing pressure, crystalline Si transforms from the diamond phase through a more highly-coordinated metallic β-tin phase, and then to a hexagonal phase. Such polymorphic phase transitions are usually accompanied by interesting changes in electronic properties. Similar to crystalline silicon, amorphous Si is also known to exhibit polymorphism. Interestingly at equilibrium, liquid Si is metallic and is denser than its crystalline counterpart, very similar to the case with water. In spite of the widespread use of Si in industry, the nature of the transition of the dense metallic liquid to an open network semiconducting solid is poorly understood.
Panchapakesan Ganesh, a postdoctoral fellow at Carnegie, has explored the structures of liquid and supercooled liquid Si using first-principles molecular-dynamics simulations, which allow the most realistic predictions for strutural properties at high pressures and temperatures. The method is unhindered by the intrinsic inaccuracy of phenomenological potentials, and has the ability to accurately capture the chemical nature of the atoms in the simulation. In the simulation, the thermodynamic pressure as a function of temperature was determined at different volumes. A "van der Waals loop" in a pressure-volume isotherm (at constant temperature) occurs when a region of positive slope interrupts the generally negative slope of the isotherm. 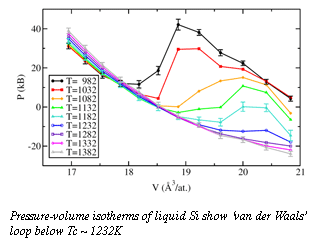 This positive slope corresponds to negative isothermal compressibility, which in the real physical system would lead to a separation of the coexisting phases. The presence of the van-der Waals loop in the pressure-volume isotherm (Fig. 1) clearly shows the presence of a liquid-liquid thermodynamic phase transition in supercooled liquid Si, and indicates that the transition is first-order (critical temperature Tc ~ 1232K and pressure Pc ~ -12kB).
This positive slope corresponds to negative isothermal compressibility, which in the real physical system would lead to a separation of the coexisting phases. The presence of the van-der Waals loop in the pressure-volume isotherm (Fig. 1) clearly shows the presence of a liquid-liquid thermodynamic phase transition in supercooled liquid Si, and indicates that the transition is first-order (critical temperature Tc ~ 1232K and pressure Pc ~ -12kB).
This work shows that the two coexisting polymorphic liquid phases are the high-density liquid (HDL), which can be considered as a metastable extension of the high-temperature equilibrium liquid (HTEL), and the low-density liquid (LDL) which is tetracoordinated and has a more open structure, like that of semiconducting solid Si. The high degree of orientational order in the open, tetrahedrally coordinated LDL Si compared to the disordered HDL Si has been proposed as the driving force behind this phase transition. The simulations also show that HDL Si is metallic, similar to liquid Si, while LDL Si has a semimetallic character, close to that of semiconducting crystalline Si.
This work has been published in Physical Review Letters [Ganesh and Widom, Phys. Rev. Lett., 102, 075701 (2009)].
