 The chemical combination of noble (platinum group) metals with elemental nitrogen represents a significant synthetic challenge, due to the inert nature of both the metal and the nitrogen-nitrogen triple bond. Recent high pressure work has shown, however, that a number of MN2 compounds (M = platinum group metal) are stable in the diamond anvil cell at elevated pressures. As a result of the difficulties involved in the solution and refinement of crystal structures from diffraction data that are dominated by scattering from the metal, the structures of the MN2 compounds have been the subject of debate and controversy.
The chemical combination of noble (platinum group) metals with elemental nitrogen represents a significant synthetic challenge, due to the inert nature of both the metal and the nitrogen-nitrogen triple bond. Recent high pressure work has shown, however, that a number of MN2 compounds (M = platinum group metal) are stable in the diamond anvil cell at elevated pressures. As a result of the difficulties involved in the solution and refinement of crystal structures from diffraction data that are dominated by scattering from the metal, the structures of the MN2 compounds have been the subject of debate and controversy.
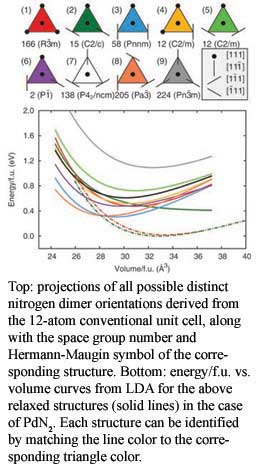
In order to provide some guidance toward the ground state crystal structures of these novel phases and a rationale for the pyrite-type structure observed for PtN2 and PdN2, a team from Livermore and Carnegie has reported a detailed theoretical study of some of the candidate structures of the platinum group metal nitrides. In this work, the thermodynamic stabilities of various possible phases of the nitrides of the Pt-metal elements were systematically studied using density functional theory. The results show that for the nitrides of Rh, Pd, Ir and Pt, two new crystal structures, in which the metal ions occupy simple tetragonal lattice sites, have lower formation enthalpies at ambient  conditions than any previously proposed structures. For PtN2, this region of stability extends to 17 GPa. These calculations also showed at this pressure, the simple tetragonal PtN2 structures are also thermodynamically stable with respect to phase separation The calculated synthesis pressures are approximately half the pressures required in the laboratory for the synthesis of the noble metal nitrides, indicating the existence of significant kinetic barriers to noble metal-dintitrogen reactivity. [Aberg, et al., Phys. Rev. Lett., 100, 095501 (2008)].
conditions than any previously proposed structures. For PtN2, this region of stability extends to 17 GPa. These calculations also showed at this pressure, the simple tetragonal PtN2 structures are also thermodynamically stable with respect to phase separation The calculated synthesis pressures are approximately half the pressures required in the laboratory for the synthesis of the noble metal nitrides, indicating the existence of significant kinetic barriers to noble metal-dintitrogen reactivity. [Aberg, et al., Phys. Rev. Lett., 100, 095501 (2008)].
