 Only a small fraction of our planet’s total carbon budget is found at the surface. In fact, Earth’s mantle is thought to be the largest carbon reservoir. Carbonates, and in particular ferromagnesite ((Mg,Fe)CO3), are likely candidates for deep-Earth carbon storage and therefore play a key role in the deep carbon cycle. The behavior of these carbonates at the high pressure and temperature conditions of Earth’s interior is therefore of great interest for understanding global carbon cycling and storage.
Only a small fraction of our planet’s total carbon budget is found at the surface. In fact, Earth’s mantle is thought to be the largest carbon reservoir. Carbonates, and in particular ferromagnesite ((Mg,Fe)CO3), are likely candidates for deep-Earth carbon storage and therefore play a key role in the deep carbon cycle. The behavior of these carbonates at the high pressure and temperature conditions of Earth’s interior is therefore of great interest for understanding global carbon cycling and storage.
A new paper, appearing in an upcoming issue of Nature Communications, reports unequivocal experimental evidence for tetrahedrally coordinated carbon in high pressure carbonates, obtained by combined experimental and theoretical studies carried out by a group including former CDAC Student and Partner Wendy Mao (Stanford University) and CDAC Scientist Zhenxian Liu (NSLS).
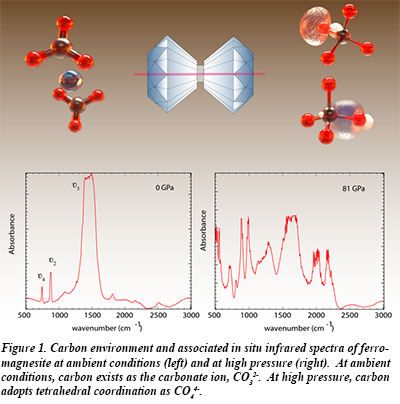
With the combination of in situ synchrotron infrared spectroscopic measurements carried out at high pressure, and ab initio calculations on the ferromagnesite structure, it was possible to identify a unique vibrational signature present only in the high-pressure phase, and thus a new carbon-oxygen bond forms in ferromagnesite under pressure. The new vibrational signature is assigned to tetrahedrally coordinated carbon atoms with asymmetric C-O bonds.
 Ferromagnesite represents an important rock-forming mineral, fundamentally distinct from silicates in Earth’s crust. At low pressure, carbon bonds to three oxygen atoms, while silicon bonds to four. Tetrahedrally coordinated carbonates likely exhibit dramatically different behavior compared to three-fold coordinated carbonates, including altered reactivity with other mantle phases and changes in chemical melt properties. This bonding behavior could therefore have significant implications for carbon reservoirs and fluxes, as well as for our understanding of the global geodynamic carbon cycle [E. Boulard et al., Tetrahedrally coordinated carbonates in Earth’s lower mantle, Nature Comm. (2015)].
Ferromagnesite represents an important rock-forming mineral, fundamentally distinct from silicates in Earth’s crust. At low pressure, carbon bonds to three oxygen atoms, while silicon bonds to four. Tetrahedrally coordinated carbonates likely exhibit dramatically different behavior compared to three-fold coordinated carbonates, including altered reactivity with other mantle phases and changes in chemical melt properties. This bonding behavior could therefore have significant implications for carbon reservoirs and fluxes, as well as for our understanding of the global geodynamic carbon cycle [E. Boulard et al., Tetrahedrally coordinated carbonates in Earth’s lower mantle, Nature Comm. (2015)].
