 A international group including Zhenxian Liu (Carnegie) and CDAC Partner Yangzhang Ma (Texas Tech University) has discovered a new chemical compound that consists of a single element―boron. Chemical compounds are conventionally defined as substances consisting of two or more elements, but these results show that at high pressure and temperature, the boron atoms in the structure can assume two distinct forms that bond together to create a novel compound called boron boride, or gamma-B28.
A international group including Zhenxian Liu (Carnegie) and CDAC Partner Yangzhang Ma (Texas Tech University) has discovered a new chemical compound that consists of a single element―boron. Chemical compounds are conventionally defined as substances consisting of two or more elements, but these results show that at high pressure and temperature, the boron atoms in the structure can assume two distinct forms that bond together to create a novel compound called boron boride, or gamma-B28.
Because of its low mass, high strength, and response to neutron irradiation, boron has important applications in technology, including nuclear engineering and in extreme environments. The present work builds on the discovery of superconductivity in elemental boron in 2001 by researchers at the Geophysical Laboratory [M. Eremets et al., Science, 293, 272-274 (2001)], and the first observation of the transition to the newly discovered structure [Y. Ma et al., Phys. Rev. B, 67, 174116 (2003)]. That study revealed superconductivity with a relatively high transition temperature for an element, but the underlying structure and mechanism have remained unexplained. The current structural and spectroscopic work provides an important step toward understanding the transition to superconductivity in boron under pressure. 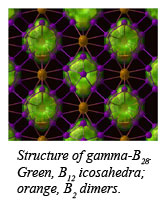 Under compression, the B12 icosahedra present in the ambient pressure phase contract, leaving two boron atoms in the inter-icosahedral voids to form a B2 dimer, leading to a denser structure (Fig. 1).
Under compression, the B12 icosahedra present in the ambient pressure phase contract, leaving two boron atoms in the inter-icosahedral voids to form a B2 dimer, leading to a denser structure (Fig. 1).
Samples of the material were prepared in the laboratory of Yingwei Fei at the Geophysical Laboratory by Y. Ma and at Stony Brook University by co-workers from Florida International University, Stony Brook University and University of Paris XIII. Spectroscopic (U2A) and diffraction (X17C) work was then carried out at the NSLS by groups headed by Z. Liu and Y. Ma, respectively. The experimental team collaborated with a group that included theorist and lead author Artem Oganov (Stony Brook University), and colleagues from ETH Zurich, University of Milan and Jilin University, which was able to predict the crystal structure of the high pressure phase and provide a rationale for its formation at high pressure and temperature. The new phase was formed at above 12 GPa and 1400 degrees Celsius using multi-anvil techniques, and is stable upon quenching to ambient conditions. Computations show that this phase should be stable between approximately 19 and 89 GPa.
The work is detailed in the latest issue of Nature [Oganov et al., Nature, doi:10.1038/nature07736 (2009)].
