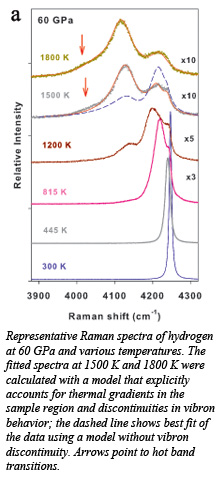
 Novel behavior of element one, hydrogen, induced by high P-T conditions has been observed at Carnegie by in situ Raman spectroscopy. The team studied the behavior of the symmetric stretching vibrational mode (molecular vibron) of hydrogen up to ~140 GPa and more than 1500 K and found unexpectedly large discontinuous softening of the vibron band over the pressure range of 30-140 GPa when the fluid phase appears at increasing temperatures. Concurrently the roton bands were found to broaden and disappear. These changes indicate that the bonding is strongly altered in hot, dense fluid hydrogen. An account of the work has been published in the Proceedings of the National Academy of Sciences [Subramanian et al., Proc. Nat. Acad. Sci., 108 6014-6019 (2011)].
Novel behavior of element one, hydrogen, induced by high P-T conditions has been observed at Carnegie by in situ Raman spectroscopy. The team studied the behavior of the symmetric stretching vibrational mode (molecular vibron) of hydrogen up to ~140 GPa and more than 1500 K and found unexpectedly large discontinuous softening of the vibron band over the pressure range of 30-140 GPa when the fluid phase appears at increasing temperatures. Concurrently the roton bands were found to broaden and disappear. These changes indicate that the bonding is strongly altered in hot, dense fluid hydrogen. An account of the work has been published in the Proceedings of the National Academy of Sciences [Subramanian et al., Proc. Nat. Acad. Sci., 108 6014-6019 (2011)].
Such experiments to elucidate the properties of hydrogen and its isotopes at high P-T conditions are important for testing predicted phase changes in the fluid phase and in the vicinity of the melting curve. The melting point is predicted to rise gradually to ~1000 K with increasing pressure up to ~100 GPa and then decrease at higher pressures. Basic thermodynamics principles imply then that the hot fluid is denser than the corresponding solid at pressure beyond above the melting curve turn over. 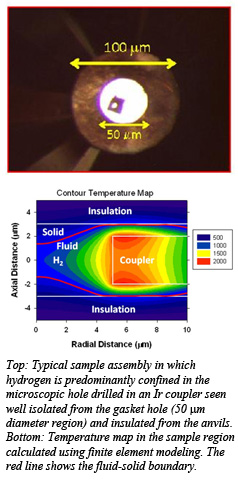 This can happen due to changes in the nature of the intermolecular and intramolecular bonding in the fluid phase. A fluid-fluid phase transition line from a non-conducting molecular phase to a disassociated conducting phase also predicted in this P-T regime, examines these models. Experimental efforts to verify these have been sparse partly because at elevated temperatures, confinement of dense hydrogen for long measurement times is challenging on account of its high chemical reactivity and diffusivity.
This can happen due to changes in the nature of the intermolecular and intramolecular bonding in the fluid phase. A fluid-fluid phase transition line from a non-conducting molecular phase to a disassociated conducting phase also predicted in this P-T regime, examines these models. Experimental efforts to verify these have been sparse partly because at elevated temperatures, confinement of dense hydrogen for long measurement times is challenging on account of its high chemical reactivity and diffusivity.
The Carnegie team successfully resolved this high P-T confinement problem by carefully locking the hydrogen in a microscopic hole (5-8 μm diameter) drilled in an IR laser absorbing iridium foil of thickness ~ 4 μm present sandwiched in an inert buffer layer isolating the two diamond anvils. To reduce the overall experiment time, a virtual experiment control and data acquisition system has also been developed for performing fully automated Raman spectroscopy  measurements while samples are laser heated. These new developments have enabled studying the vibron behavior at extreme conditions over multiple heating-cooling cycles.
measurements while samples are laser heated. These new developments have enabled studying the vibron behavior at extreme conditions over multiple heating-cooling cycles.
These results are consistent with the existence of a high P-T transformation in the fluid related to the presence of a temperature maximum in the melting line as a function of pressure. The fluid phase formed at pressures above the melting maximum is characterized by short-range orientational order and interatomic interactions that differ from those of the fluid at lower pressures.
