 Molecular oxygen (O2) changes its form dramatically with compression, transforming to a solid with spectacular colors, and eventually becoming metallic and superconducting at sufficiently high pressures. The underlying mechanism for these remarkable phenomena has been a source of debate for decades, in particular the origin of the recently discovered molecular cluster (O2)4 in the dense solid, red phase. Yue Meng (HPCAT), and researchers from the Geophysical Laboratory, University of Chicago, University of Saskatchewan, and the National Synchrotron Light Source found that under pressure
Molecular oxygen (O2) changes its form dramatically with compression, transforming to a solid with spectacular colors, and eventually becoming metallic and superconducting at sufficiently high pressures. The underlying mechanism for these remarkable phenomena has been a source of debate for decades, in particular the origin of the recently discovered molecular cluster (O2)4 in the dense solid, red phase. Yue Meng (HPCAT), and researchers from the Geophysical Laboratory, University of Chicago, University of Saskatchewan, and the National Synchrotron Light Source found that under pressure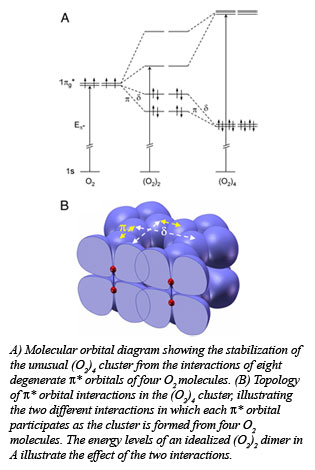 the individual molecules interact through their outermost, highest energy molecular orbitals. This pairing interaction brings four oxygen molecules together to form discrete (O2)4clusters at a pressure of about 100, 000 times atmospheric pressure, or 10 gigapascals. With increasing pressure, the effective radial extent of the molecular orbitals on individual molecules increases, promoting the interaction of unpaired electrons on adjacent molecules.
the individual molecules interact through their outermost, highest energy molecular orbitals. This pairing interaction brings four oxygen molecules together to form discrete (O2)4clusters at a pressure of about 100, 000 times atmospheric pressure, or 10 gigapascals. With increasing pressure, the effective radial extent of the molecular orbitals on individual molecules increases, promoting the interaction of unpaired electrons on adjacent molecules.
Using diamond anvil cell methods along with the newly developed high pressure inelastic x-ray scattering technique, the group was able to track the behavior of the relevant molecular orbitals on the oxygen molecules with compression. The fact that the energy of the scattered x-rays increased with increasing pressure indicated that the bonding character of the highest energy molecular orbitals was changing, as illustrated in the Figure. Theoretical studies of the energetics of formation of the (O2)4 clusters provide a rationale for the formation of the tetramolecular unit as opposed to the dimer (O2)2. The work suggests that interactions of a similar nature, which are well known in organic chemistry, could occur between (O2)4 clusters at higher pressures, leading to still other, as yet undiscovered phases [Meng, et al., Proc. Nat. Acad. Sci., 105, 11640-11644 (2008)]
