SiO2 glass is a prototypical network forming glass with a high degree of intermediate range order (IRO). The open structure of SiO2 glass makes it highly compressible, with molar volumes decreasing sharply with pressure 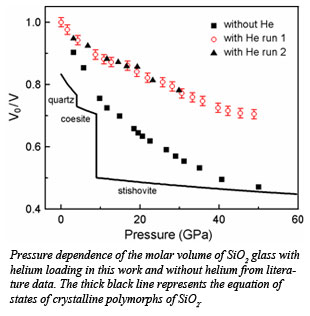 exceeding those of crystalline polymorphs of quartz and coesite at around 20 GPa and approaching to that of dense stishovite at 40 GPa. When SiO2 glass is loaded with helium under pressure, however, it becomes much less compressible. The stiffness may be a result of helium entering in the voids in SiO2 glass under pressure. The results are published in a recent publication in the Proceedings of the National Academy of Sciences by Shen et al.
exceeding those of crystalline polymorphs of quartz and coesite at around 20 GPa and approaching to that of dense stishovite at 40 GPa. When SiO2 glass is loaded with helium under pressure, however, it becomes much less compressible. The stiffness may be a result of helium entering in the voids in SiO2 glass under pressure. The results are published in a recent publication in the Proceedings of the National Academy of Sciences by Shen et al.
Incorporation of helium in SiO2 glass is verified by x-ray diffraction and optical Raman spectroscopy. The tray data, measured at HPCAT with a diamond anvil cell, show that the first sharp diffraction peak (FSDP) position of SiO2 glass in helium medium remains essentially the same under pressures up to 18.6 GPa, which is in stark difference from that of SiO2 glass without pressure medium, where the large increase in density under pressure has been attributed to significant modification of the IRO structure manifested by a drastic change in the FSDP in the structure factor.  The observed preservation of the FSDP may reflect an effect of dissolved He on the structure of SiO2 glass and, therefore, compression mechanisms.
The observed preservation of the FSDP may reflect an effect of dissolved He on the structure of SiO2 glass and, therefore, compression mechanisms.
Experiments on GeO2 glass and SiO2 glass with H2 as a pressure medium do not show this stiffness effect, implying that size plays a major role in understanding the effect. Any elements or molecules with kinetic diameters larger than that of H2 (0.29 nm) cannot enter the voids of the glass in a large amount. So far, the effect of helium on the structure and compression of SiO2 glass is found to be unique, because of the combination of controlling factors including the small kinetic diameter of helium, the high degree of IRO with large rings in SiO2 glass, and the large fraction of interstitial solubility sites occupied by helium [Shen et al., Proc. Nat. Acad. Sci., 108, 6004-6007 (2011)].
